In the ongoing battle against parasitic worms, anthelmintic drugs have been our stalwart defenders, safeguarding human health against the scourge of helminthiasis. Among these pharmaceutical champions, mebendazole has stood out for its efficacy, safety, and broad-spectrum activity. However, as we have harnessed these drugs to combat soil-transmitted helminth (STH) infections, a new challenge has insidiously emerged: anthelmintic resistance. This phenomenon, once confined to the realm of veterinary medicine, is now a growing concern for human health, threatening to undermine decades of progress in disease control and public health initiatives.
The evolution of anthelmintic resistance is a complex interplay of biological, environmental, and human factors. From the genetic mechanisms that enable a worm to survive a dose of mebendazole, to the socio-economic factors that drive sub-optimal dosing practices, each contributes to the tapestry of resistance. This article delves into the multifaceted nature of anthelmintic resistance, examining the confounding factors that affect drug efficacy and the drivers that fuel the development of resistant strains. Through a critical analysis of recent reports on drug resistance and a discussion on the challenges of defining resistance in hookworms and other parasites, we aim to provide a comprehensive overview of the current landscape.
The use of mebendazole and other anthelmintics in treatment protocols is at a crossroads. With resistance looming, the need for innovative approaches to drug administration, including targeted deworming protocols and the judicious use of pharmaceuticals, has never been more pressing. This article also explores the concept of One Health, an integrative effort that recognizes the interconnectedness of people, animals, plants, and their shared environment, in the context of anthelmintic drug resistance. By encouraging responsible use among stakeholders and implementing effective diagnostic tools and surveillance measures, we can hope to sustain the efficacy of mebendazole and other essential drugs.
As we stand on the precipice of potential crisis, this article not only seeks to highlight the challenges posed by anthelmintic resistance but also to chart a course forward. Through research priorities and recommendations for standard operating procedures, we endeavor to equip researchers, clinicians, and policy-makers with the knowledge and tools necessary to counteract the rise of resistance. The battle against parasitic worms is far from over, but with informed strategies and collective action, we can continue to protect and improve human health around the globe.
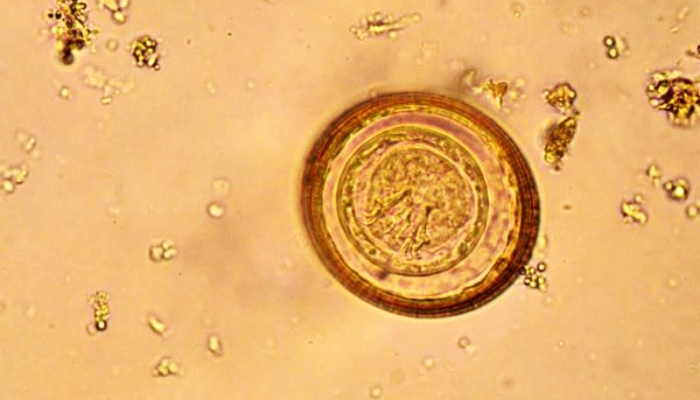
Confounding Factors Affecting Anthelmintic Efficacy
As we delve into the nuances of anthelmintic resistance, it becomes imperative to first understand the myriad of confounding factors that influence the efficacy of these drugs. Anthelmintic efficacy is not a static measure but a dynamic outcome influenced by a confluence of biological, pharmacological, and environmental variables. These factors, often interrelated, can obscure the true effectiveness of treatments like mebendazole and complicate the assessment of resistance development, highlighting the importance of monitoring drug efficacy and conducting drug efficacy trials.
Biological Variability
At the heart of the matter is biological variability among parasite populations. Genetic diversity within and between species of helminths can lead to variable drug susceptibility. For instance, mutations in specific genes may confer resistance to certain anthelmintics, allowing some individuals to survive treatment and reproduce, thereby propagating the resistance alleles, including bz resistance. This genetic variation is a natural barrier to the universal efficacy of any anthelmintic and underscores the challenge of parasite control using the same drug across different populations.
Drug Administration and Absorption
The manner in which a drug is administered, as well as the host’s ability to absorb it, significantly impacts its effectiveness. Factors such as the formulation of the drug, the timing of administration relative to food intake, and the physiological condition of the gastrointestinal tract can all affect the bioavailability of anthelmintics. Sub-optimal absorption may result in insufficient drug concentrations at the site of action, diminishing efficacy without necessarily indicating true resistance, a key consideration in morbidity control.
Environmental and Socio-economic Factors
Environmental conditions and socio-economic factors play crucial roles in anthelmintic efficacy. Poor sanitation, high population density, and limited access to healthcare resources can facilitate the transmission of helminth infections and hinder the implementation of effective treatment protocols. Furthermore, socio-economic constraints may lead to the use of sub-optimal doses or the reuse of drugs, practices that can encourage the development of drug resistance in humans.
Host Immune Response
The host’s immune response to helminth infection also influences the outcome of anthelmintic treatment. An effective immune response can complement the action of anthelmintics, reducing parasite burdens and potentially masking sub-optimal drug efficacy. Conversely, an impaired immune system may allow for higher parasite burdens and increased exposure to the drug, setting the stage for the selection of resistant strains.
Interaction with Other Medications
The concurrent use of other medications can affect the pharmacokinetics and pharmacodynamics of anthelmintics. Drug interactions may enhance or inhibit the metabolism of anthelmintics, altering their efficacy and potentially leading to erroneous conclusions about resistance.
Understanding these confounding factors is critical for accurately assessing the effectiveness of anthelmintic treatments and for designing studies to monitor resistance. It highlights the complexity of interpreting data on drug efficacy and underscores the need for meticulous experimental design and analysis. As we move forward, addressing these challenges will be paramount in our efforts to sustain the efficacy of anthelmintics like mebendazole and to adapt our strategies in the face of emerging resistance.
Contributing Factors to the Development of Drug Resistance
The development of drug resistance in helminths, a growing threat to public health efforts against parasitic infections, is influenced by a constellation of factors. These factors, ranging from the genetic to the environmental, interact in complex ways to undermine the efficacy of anthelmintics, including mebendazole. Understanding these contributing elements is crucial for devising strategies to mitigate resistance and preserve the utility of existing treatments.
Genetic Selection
The cornerstone of resistance development is genetic selection. Every use of an anthelmintic imposes selective pressure on the helminth population. Those parasites harboring genetic mutations that confer survival advantages under drug exposure are more likely to survive and reproduce. Over time, these resistant strains can become predominant, rendering treatments less effective. The frequency and intensity of drug application play significant roles in accelerating this process.
Overuse and Misuse of Anthelmintics
The overuse and misuse of anthelmintics significantly contribute to the development of resistance. In human medicine, this may manifest as incorrect dosing, either due to inaccuracies in weight estimation or non-adherence to prescribed treatment courses. In veterinary and agricultural contexts, the prophylactic mass treatment of livestock, often without confirmation of infection, exacerbates the issue. Such practices not only select for resistant parasites but also reduce the effective lifespan of anthelmintic drugs.
Lack of Rotation and Monotherapy
A lack of rotation between different classes of anthelmintics and the reliance on monotherapy are other critical factors. Using a single drug or class of drugs for extended periods increases the likelihood of resistance development. In contrast, rotating drugs with different mechanisms of action can help manage and slow the spread of resistance.
Environmental Persistence
The environmental persistence of anthelmintics also plays a role. Residues from animal treatments can contaminate soil and water, exposing free-living stages of helminths to sub-lethal doses of drugs. This low-level exposure can facilitate the selection of resistant individuals, contributing to the broader problem of resistance in the environment.
Poor Diagnostics and Monitoring
The lack of sensitive diagnostic tools and effective monitoring programs compounds the problem. Without accurate diagnostics, infections may be improperly treated or go unnoticed until they become severe, necessitating more aggressive treatment that further selects for resistance. Additionally, a lack of surveillance impedes the early detection of resistance, delaying the implementation of corrective measures.
Global Movement and Livestock Trade
Global movement of people and animals accelerates the spread of resistant strains. The international trade of livestock can quickly disseminate helminths with resistant alleles across borders, spreading resistance from regions where anthelmintic use is intensive to those previously unaffected.
Socio-Economic Factors
Finally, socio-economic factors influence resistance development. Limited access to healthcare and veterinary services, coupled with a lack of education on proper drug use, can lead to the inappropriate application of anthelmintics. In resource-limited settings, the economic pressure to maximize agricultural productivity may prioritize short-term gains over long-term sustainability, promoting practices that favor resistance development.
Addressing the multifaceted nature of anthelmintic resistance requires a comprehensive approach, integrating improved drug management strategies, enhanced diagnostic capabilities, and international cooperation. By acknowledging and tackling these contributing factors, we can devise more effective interventions to slow the evolution of resistance and extend the efficacy of drugs like mebendazole.
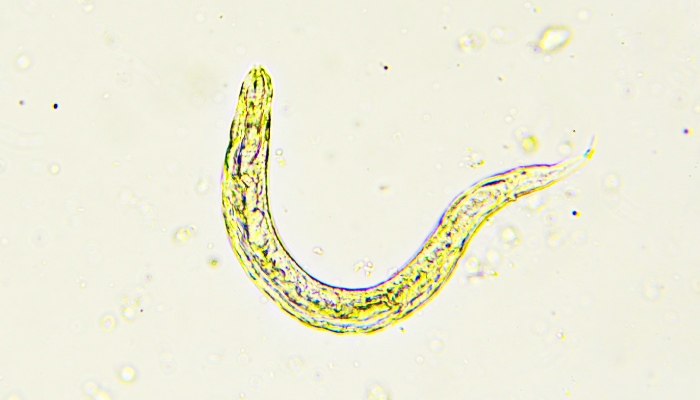
The Use of Anthelmintics in Sub-Optimal Doses
The administration of anthelmintics in sub-optimal doses represents a critical and widespread issue in the fight against parasitic infections, significantly contributing to the development and propagation of drug resistance. Sub-optimal dosing occurs when the amount of drug administered is insufficient to kill or inhibit the target parasite population effectively. This scenario can arise from various causes, including inaccurate dosing, poor drug formulation, and intentional reduction of doses to save costs. The consequences of such practices are profound, not only undermining individual treatment outcomes but also facilitating the broader emergence of resistance.
Causes of Sub-Optimal Dosing
Inaccurate Dosing: One of the primary causes of sub-optimal dosing is the inaccurate estimation of the appropriate drug dosage, which can occur due to errors in weighing or measuring the medication or incorrect assessment of the patient’s weight. In veterinary medicine, this issue is compounded by the challenge of dosing animals in a herd or flock, where individual variations are often overlooked in favor of group treatment strategies.
Economic Constraints: In both human and veterinary contexts, economic constraints can lead to the intentional use of lower-than-recommended doses. This is particularly prevalent in resource-limited settings where the cost of medications can be prohibitive. The goal is often to stretch limited resources, but the long-term consequence is the potential for resistance development, which can ultimately increase costs due to the need for more potent or multiple treatments.
Poor Drug Formulation and Absorption: Even when doses are correctly calculated and administered, poor drug formulation can lead to sub-optimal bioavailability. Similarly, factors affecting the patient’s absorption of the drug, such as malnutrition or gastrointestinal disease, can reduce effective drug concentrations at the site of action, inadvertently resulting in sub-optimal dosing.
Consequences of Sub-Optimal Dosing
Selection Pressure for Resistance: Sub-optimal dosing exerts selective pressure on parasitic populations by exposing them to drug concentrations that are insufficient to ensure complete eradication. This environment allows parasites with even slight genetic advantages in drug resistance to survive, reproduce, and gradually become the dominant strain.
Reduced Drug Efficacy: In the short term, sub-optimal dosing can lead to reduced efficacy of treatment, resulting in persistent infections and the need for repeated or alternative treatments. This not only impacts patient health but also increases the risk of transmission of resistant parasites.
Economic and Public Health Impact: The broader economic and public health impacts of sub-optimal dosing are significant. In agriculture, for instance, resistant infections can lead to decreased productivity and increased costs associated with treatment failures. For human health, the implications include increased morbidity, mortality, and healthcare costs associated with managing persistent or resistant infections.

Addressing the Challenge
To mitigate the risks associated with sub-optimal dosing, efforts must be directed towards improving drug administration practices, enhancing the accuracy of dosing, and educating healthcare providers and patients on the importance of adhering to recommended dosages. Additionally, the development of more effective formulations and delivery systems can help ensure that therapeutic drug levels are achieved and maintained.
Investing in research to understand the pharmacokinetics and pharmacodynamics of anthelmintics in different populations and under various conditions can also provide valuable insights. Such knowledge can guide the refinement of dosing strategies to maximize efficacy and minimize the risk of resistance development.
The use of anthelmintics in sub-optimal doses presents a complex challenge, intertwined with issues of accessibility, education, and drug development. Addressing this issue is critical for sustaining the effectiveness of current treatments and safeguarding against the escalation of drug-resistant parasitic infections.
Genetic Mechanisms Behind Drug Resistance
The emergence of drug resistance in helminths, a significant concern for global health, is underpinned by complex genetic mechanisms. Understanding these mechanisms is crucial for developing strategies to combat resistance and ensure the continued efficacy of anthelmintic drugs like mebendazole. Resistance arises when genetic variations within the parasite population confer an advantage under the selective pressure exerted by anthelmintic treatments, allowing these resistant individuals to survive, reproduce, and eventually dominate.
Genetic Mutations
The foundation of drug resistance often lies in genetic mutations. These mutations can affect various aspects of the parasite’s biology, such as the drug’s target site, metabolic pathways that inactivate the drug, or mechanisms that expel the drug from the parasite’s cells. For example, mutations in genes coding for specific receptors or enzymes that are targets of the drug can alter the binding site, rendering the drug less effective or completely ineffective.
Gene Amplification
Another genetic mechanism contributing to resistance is gene amplification, where multiple copies of a gene enhance the production of a target enzyme or protein. This overproduction can negate the drug’s effect, as there is more target than the drug can inhibit or bind to, allowing the parasite to survive despite the presence of the anthelmintic.
Epigenetic Changes
Epigenetic modifications, which influence gene expression without altering the DNA sequence, also play a role in drug resistance. These changes can lead to the upregulation of genes involved in drug detoxification or efflux, increasing the parasite’s ability to withstand anthelmintic exposure. Unlike genetic mutations, epigenetic changes are reversible and can occur rapidly, offering a quick adaptation mechanism to environmental pressures, including drug treatment.
Horizontal Gene Transfer
In some cases, resistance can spread through populations via horizontal gene transfer, though this mechanism is more commonly observed in bacteria than in eukaryotic organisms like helminths. This process involves the transfer of genetic material between individuals, disseminating resistance genes across a population without the need for resistant individuals to outcompete susceptible ones through reproduction.
Genetic Diversity and Population Structure
The genetic diversity and population structure of helminths can influence the spread and maintenance of resistance. High genetic variability within a population can increase the likelihood of resistance alleles arising. Furthermore, the population structure, influenced by factors such as reproductive strategy (e.g., self-fertilization vs. cross-fertilization) and migration patterns, affects how quickly resistance alleles spread through the population.
Understanding the genetic mechanisms behind drug resistance illuminates the challenges in managing and preventing resistance. It underscores the importance of using anthelmintics judiciously and highlights the need for ongoing surveillance to detect the emergence of resistance early. Furthermore, insights into these mechanisms are invaluable for the development of new drugs designed to circumvent existing resistance mechanisms and for refining treatment strategies to minimize the selection pressure that drives the development of resistance.
Drivers of Resistance
The proliferation of drug resistance in helminth populations is not a phenomenon occurring in isolation. Instead, it is driven by a complex interplay of factors that extend beyond the genetic predispositions of these parasites. Understanding the drivers of resistance is pivotal for developing comprehensive strategies to mitigate its spread and ensure the longevity of anthelmintic drugs like mebendazole. Here, we explore the multifaceted drivers that contribute to the development and propagation of resistance.
Intensive and Inappropriate Use of Anthelmintics
The intensive and inappropriate use of anthelmintics stands out as a primary driver of resistance. This includes both the overuse and misuse of these drugs, such as treating entire populations indiscriminately (mass drug administration) without confirming the presence of infection or using drugs in doses that are not aligned with the recommended guidelines. Such practices exert strong selective pressure on helminth populations, favoring the survival and reproduction of resistant individuals.
Inadequate Rotation of Drug Classes
A lack of rotation among different classes of anthelmintics can also drive the development of resistance. Relying on a single drug or drugs with similar mechanisms of action for extended periods allows parasites with resistance mechanisms specific to that action mode to thrive. Rotating drugs with different mechanisms of action can help prevent the establishment and spread of resistant strains by diversifying the selection pressure.
Environmental and Ecological Factors
Environmental and ecological factors play significant roles in the dynamics of resistance. The persistence of drug residues in the environment, for instance, can expose parasites to sub-lethal doses of anthelmintics, promoting the selection of resistant traits. Additionally, the movement of hosts, whether through migration or the trade of livestock, can facilitate the spread of resistance genes across populations and geographic regions.
Host Immunity and Behavior
The host’s immune response and behavior can indirectly influence the development of resistance. A strong immune response may reduce the parasite burden to levels where drug treatment is more effective, potentially minimizing the selection for resistant parasites. Conversely, behaviors that lead to increased exposure to infected environments or inadequate compliance with treatment regimens can enhance the risk of resistance.
Economic and Social Factors
Economic and social factors are critical drivers of resistance. In resource-limited settings, the cost of drugs and access to veterinary or medical services can lead to the use of sub-optimal dosages or reliance on cheaper, less effective treatments. Moreover, a lack of awareness or education regarding the proper use of anthelmintics can contribute to practices that favor the development of resistance.
Policy and Regulatory Frameworks
The regulatory landscape governing the use of anthelmintics can either mitigate or exacerbate the problem of resistance. Strong policies that promote the judicious use of these drugs, coupled with effective surveillance and monitoring programs, can help manage resistance. However, the absence of such frameworks, or their inadequate enforcement, can leave room for practices that contribute to the spread of resistance.
Addressing the drivers of anthelmintic resistance requires a multifaceted approach that encompasses judicious drug use, improved diagnostics, education and awareness campaigns, and robust policy frameworks. By tackling these drivers, we can aim to sustain the effectiveness of current anthelmintic drugs and safeguard public health efforts against the burden of helminth infections.

Problems of Defining Drug Resistance in Hookworms
Defining drug resistance in hookworms, a significant cause of morbidity in tropical and subtropical regions, presents unique challenges. These challenges stem from biological, diagnostic, and epidemiological complexities that obscure the assessment and understanding of resistance. As hookworms continue to burden human populations, particularly where sanitation is inadequate, the precise definition of drug resistance becomes critical for effective control and treatment strategies. This exploration into the problems of defining drug resistance in hookworms sheds light on the hurdles facing researchers and healthcare providers.
Biological Complexity
Hookworms exhibit a high degree of biological complexity, with life cycles that involve both free-living and parasitic stages. This complexity complicates the identification of resistance, as the effectiveness of anthelmintics can vary across different stages of the hookworm’s lifecycle. Additionally, the genetic mechanisms underpinning resistance may be multifactorial, making it difficult to pinpoint specific markers of resistance.
Variability in Drug Efficacy
The efficacy of anthelmintics against hookworms can vary significantly depending on a host of factors, including the specific species of hookworm, the drug’s pharmacokinetics, and the host’s immune response. This variability introduces ambiguity in determining whether a treatment failure is due to drug resistance or other factors affecting drug effectiveness.
Diagnostic Limitations
Current diagnostic tools for detecting hookworm infections and assessing anthelmintic efficacy are limited in sensitivity and specificity. Traditional methods, such as fecal egg count reduction tests (FECRT), are commonly used but can be unreliable in detecting low-level infections or subtle changes in drug efficacy. Without precise and sensitive diagnostic techniques, accurately identifying and quantifying resistance in hookworm populations remains a significant challenge.
Epidemiological Factors
The epidemiology of hookworm infections, characterized by high rates of re-infection in endemic areas, complicates the assessment of drug resistance. Distinguishing between treatment failure due to resistance and re-infection from environmental exposure is challenging without longitudinal studies and sophisticated epidemiological analyses. Furthermore, the widespread use of mass drug administration (MDA) programs for hookworm and other soil-transmitted helminths (STHs) adds another layer of complexity in monitoring resistance trends over time.
Lack of Standardized Definitions
A standardized definition of drug resistance in the context of hookworms and other STHs is lacking. Without universally accepted criteria for identifying and classifying resistance, comparing findings across studies becomes problematic. This lack of standardization hampers the development of global strategies for resistance management and control.
Implications for Control Programs
The difficulties in defining and detecting drug resistance in hookworms have direct implications for control programs. Misinterpretation of resistance can lead to inappropriate changes in treatment protocols, potentially exacerbating the problem of resistance or failing to address it effectively. Developing more accurate diagnostic tools and refining definitions of resistance are essential steps toward improving the management of hookworm infections and ensuring the continued success of anthelmintic treatment programs.
Addressing the challenges of defining drug resistance in hookworms requires a concerted effort from the scientific community, involving advances in genetic research, diagnostics, and epidemiological monitoring. By overcoming these obstacles, we can enhance our capacity to detect, understand, and combat anthelmintic resistance, safeguarding the health of affected populations worldwide.
Treatment of STH Infections
The treatment of Soil-Transmitted Helminth (STH) infections, which include infections by hookworms, roundworms (Ascaris lumbricoides), and whipworms (Trichuris trichiura), is a cornerstone of public health efforts in many parts of the world. Effective management of these infections is crucial not only for alleviating the immediate burden of disease but also for preventing the long-term sequelae that can affect growth, cognitive development, and overall quality of life. The strategy for treating STH infections encompasses a combination of anthelmintic drug administration, improvement of sanitation, and health education. Here, we focus on the pharmacological aspects of treatment, particularly the role of mebendazole and other anthelmintics.
Anthelmintic Drugs for STH Treatment
Mebendazole and albendazole are the primary drugs used for the treatment of STH infections. They are effective against the major STHs and are recommended by the World Health Organization (WHO) for both individual treatment cases and mass drug administration (MDA) programs. These drugs work by inhibiting the uptake of glucose by the helminths, leading to their eventual death and expulsion from the host’s body.
Ivermectin is another drug used in certain contexts, especially for infections involving Strongyloides stercoralis, and in areas co-endemic with filarial infections, given its efficacy against both STHs and filarial worms.
Mass Drug Administration (MDA)
MDA is a key public health strategy for reducing the burden of STH infections in endemic regions. It involves the periodic administration of anthelmintic drugs to all at-risk populations, particularly children, without prior individual diagnosis. MDA aims to reduce the prevalence of infections, decrease the worm burden, and mitigate the transmission of STHs. Mebendazole and albendazole are the most commonly used drugs in MDA programs due to their safety profile, efficacy, and the feasibility of large-scale administration.
Challenges in STH Treatment
While anthelmintic drugs are effective in reducing the burden of STH infections, several challenges persist:
- Drug Resistance: The potential development of resistance to anthelmintics, as seen in veterinary medicine, poses a significant threat to the sustainability of control programs.
- Re-Infection: In areas with poor sanitation and hygiene, the risk of re-infection after treatment is high, necessitating repeated rounds of MDA and highlighting the need for integrated approaches that include improvements in sanitation and health education.
- Coverage and Compliance: Achieving high coverage in MDA programs and ensuring compliance with treatment are essential for the success of control efforts. Logistical, cultural, and educational barriers can affect both.
- Monitoring and Evaluation: Effective monitoring and evaluation are required to assess the impact of treatment programs, detect the emergence of drug resistance, and adjust strategies accordingly.
Integrated Control Measures
For sustainable control of STH infections, pharmacological treatment must be part of an integrated strategy that includes improving water, sanitation, and hygiene (WASH) practices, health education, and nutritional support. These measures can help reduce transmission, prevent re-infection, and address the underlying determinants of STH infections.

Future Directions
Advancements in the development of new anthelmintic drugs, vaccines, and diagnostic tools are crucial for enhancing the efficacy of STH control programs. Additionally, operational research to optimize MDA strategies, understand the dynamics of drug resistance, and integrate STH control with other disease control efforts can provide valuable insights for public health interventions.
Treating STH infections effectively requires a comprehensive approach that combines immediate pharmacological interventions with long-term preventive measures. By addressing the challenges and leveraging new advances in science and medicine, we can move closer to achieving the goal of eliminating STHs as a public health problem.
Implementing Targeted Deworming Protocols
Implementing targeted deworming protocols represents a strategic shift from the traditional approach of mass drug administration (MDA) to a more nuanced method of controlling Soil-Transmitted Helminth (STH) infections. This shift aims to enhance the efficiency and sustainability of deworming efforts by focusing resources on populations at highest risk of infection and morbidity. The rationale behind targeted deworming lies in optimizing the use of anthelmintic drugs, like mebendazole, to minimize the potential for drug resistance while still effectively reducing the burden of STH infections. Here, we explore the key aspects of implementing targeted deworming protocols.
Identification of High-Risk Populations
The first step in targeted deworming is the accurate identification of high-risk groups. These typically include children, pregnant women, and individuals in occupations that expose them to high levels of environmental contamination. Geographical information systems (GIS) and epidemiological data can be utilized to map infection prevalence and identify areas where targeted interventions can have the greatest impact.
Integration with Local Health Systems
Successful implementation of targeted deworming protocols requires close integration with local health systems. This integration facilitates the distribution of anthelmintic treatments and enables the monitoring and evaluation of program outcomes. Collaborating with community health workers and leveraging existing health infrastructure can enhance the reach and effectiveness of targeted deworming initiatives.
Use of Diagnostic Tools
Advanced diagnostic tools play a crucial role in targeted deworming protocols. Unlike MDA, which does not require individual diagnosis, targeted approaches depend on the accurate detection of infections to direct resources effectively. The development and deployment of sensitive, scalable diagnostic methods are essential for identifying individuals and communities that would benefit most from deworming interventions.
Education and Community Engagement
Education and community engagement are integral to the success of targeted deworming efforts. Raising awareness about the risks of STH infections and the benefits of treatment can improve participation rates and compliance. Engaging communities in the planning and implementation of deworming protocols also fosters a sense of ownership and responsibility, further enhancing the sustainability of control efforts.
Monitoring and Resistance Management
Targeted deworming protocols necessitate robust systems for monitoring and evaluation. Regular assessment of infection prevalence, drug efficacy, and the emergence of resistance is critical for adapting strategies over time. Implementing a surveillance system that includes resistance monitoring can help ensure the continued effectiveness of anthelmintic drugs and inform adjustments to deworming protocols as needed.
Ethical Considerations
Implementing targeted deworming raises ethical considerations, particularly regarding the equitable distribution of health interventions. Ensuring that targeted approaches do not inadvertently exclude or marginalize vulnerable populations is paramount. Ethical frameworks guiding the allocation of resources and interventions can help address these concerns.
Challenges and Opportunities
While targeted deworming offers several advantages over mass drug administration, it also presents challenges. The need for diagnostic screening, the potential for stigmatization of treated individuals, and the logistical complexities of targeting interventions can pose barriers to implementation. However, with careful planning and community involvement, targeted deworming protocols can provide a more sustainable and effective approach to controlling STH infections, ultimately contributing to the goal of elimination.
Implementing targeted deworming protocols requires a multifaceted approach that balances efficiency, sustainability, and equity. By focusing on high-risk populations, integrating interventions with local health systems, and employing sensitive diagnostic tools, targeted deworming can play a crucial role in the global effort to control and eliminate STH infections.
Targeting and Timing of Mass Treatment
The strategic targeting and timing of mass treatment for Soil-Transmitted Helminth (STH) infections are crucial elements in the global effort to control and eventually eliminate these infections as public health concerns. Mass drug administration (MDA) programs, which involve the distribution of anthelmintic drugs such as mebendazole and albendazole to at-risk populations without prior individual diagnosis, have been central to these efforts. However, the effectiveness of MDA programs is significantly influenced by how well the targeting and timing of these interventions are managed. This approach involves not only identifying the most vulnerable populations but also determining the optimal times for drug administration to maximize impact and sustainability.
Identifying High-Risk Populations
Effective targeting begins with the identification of populations most at risk of STH infections. Typically, these include preschool and school-age children, women of childbearing age, and communities living in areas with poor sanitation and hygiene. High-risk populations may also be defined by epidemiological factors, such as infection prevalence rates and intensity, which can vary significantly across different regions and communities.
Timing of Drug Administration
The timing of drug administration is determined by several factors, including the life cycle of the parasites, the epidemiology of the infections, and local environmental conditions that may affect transmission rates. Generally, treatments are timed to coincide with periods of low transmission to reduce re-infection rates and maximize the period of reduced worm burden within the community. In many settings, treatments are administered annually or biannually, though the frequency may be adjusted based on the local epidemiological context.
Seasonal Considerations
In many endemic regions, STH transmission is influenced by seasonal variations in climate and human behavior. Seasonal timing of MDA can take advantage of these patterns to strategically reduce the worm burden and interrupt transmission. For example, administering treatments just before the rainy season in areas where transmission peaks with the onset of rains can be an effective strategy.
Integration with Other Health Interventions
The targeting and timing of mass treatment are often coordinated with other public health interventions to maximize impact and resource efficiency. For instance, MDA programs may be synchronized with vaccination campaigns, nutritional supplementation programs, or maternal and child health services. This integrated approach not only broadens the reach of STH control efforts but also strengthens the overall health system.
Monitoring and Evaluation
Ongoing monitoring and evaluation are essential to assess the effectiveness of MDA programs and to refine targeting and timing strategies. This includes tracking infection prevalence and intensity, assessing drug efficacy, and monitoring for potential drug resistance. Data collected through these efforts inform adjustments to the targeting and timing of treatments to ensure that MDA programs remain responsive to changing epidemiological and environmental conditions.
Challenges and Adaptations
Implementing targeted and timely mass treatment faces several challenges, including logistical constraints, ensuring community participation, and addressing concerns about drug resistance. Programs must be adaptable, capable of responding to new information about infection patterns, and sensitive to community needs and preferences.
The strategic targeting and timing of mass treatment for STH infections are dynamic processes that require continuous evaluation and adaptation. By focusing on high-risk populations and optimizing the timing of interventions, public health programs can enhance the effectiveness of MDA campaigns, reduce the burden of STH infections, and move closer to achieving global health objectives.
Developing and Promoting the Use of Effective Diagnostic Tools
The battle against Soil-Transmitted Helminth (STH) infections, a critical component of global public health initiatives, hinges not only on effective treatment strategies but also on the ability to accurately diagnose these infections. Developing and promoting the use of effective diagnostic tools are paramount to this effort, enabling the identification of infected individuals and communities, guiding treatment decisions, and monitoring the effectiveness of control programs. The advancement of diagnostic technologies and methodologies is essential for the timely detection of STH infections, the assessment of infection intensity, and the surveillance of drug resistance patterns.
Advances in Diagnostic Technologies
Recent advances in diagnostic technologies have led to the development of more sensitive and specific tools for the detection of STH infections. Molecular techniques, such as polymerase chain reaction (PCR) and loop-mediated isothermal amplification (LAMP), offer significant improvements over traditional microscopy-based methods. These molecular diagnostics can detect low-level infections that might be missed by conventional techniques, and they can differentiate between species of helminths, providing valuable epidemiological data.
Integration with Health Systems
For diagnostic tools to be effective, they must be integrated into existing health systems in a way that supports their widespread use, especially in resource-limited settings where the burden of STH infections is highest. This integration involves training healthcare workers in the use of new diagnostic technologies, ensuring the availability of necessary equipment and reagents, and establishing clear protocols for the interpretation and reporting of results.
Role in Targeted Treatment Strategies
Effective diagnostic tools are crucial for implementing targeted treatment strategies. By accurately identifying infected individuals and assessing infection intensity, healthcare providers can tailor treatment regimens to the specific needs of patients or communities, optimizing the use of anthelmintic drugs and reducing the risk of drug resistance. Furthermore, diagnostics play a key role in identifying high-risk populations for targeted interventions, enhancing the efficiency and impact of control programs.
Surveillance and Monitoring
The development and promotion of effective diagnostic tools also enhance the capacity for surveillance and monitoring of STH infections. Regular monitoring of infection prevalence and intensity, as well as the detection of emerging drug resistance, is vital for assessing the effectiveness of control strategies and making necessary adjustments. Advanced diagnostic tools enable more accurate and timely surveillance, providing the data needed to guide public health policy and programmatic decisions.
Challenges and Future Directions
Despite advances in diagnostic technologies, several challenges remain. The cost and complexity of molecular diagnostics may limit their use in low-resource settings. There is also a need for point-of-care diagnostics that can be easily deployed in community settings without specialized equipment or training. Addressing these challenges requires ongoing research and development efforts, as well as partnerships between public health organizations, research institutions, and industry.
Conclusions
In conclusion, the evolution of anthelmintic resistance poses a significant challenge to the global control of Soil-Transmitted Helminth (STH) infections and threatens to undermine the progress achieved in public health over the past decades. The development and spread of resistance are driven by a complex interplay of factors, including the misuse and overuse of anthelmintic drugs, genetic mutations within parasite populations, and environmental and socio-economic conditions. Addressing this challenge requires a multifaceted approach that encompasses the judicious use of existing anthelmintic drugs, the development and implementation of targeted deworming protocols, and the advancement of diagnostic tools for effective disease monitoring and resistance surveillance, including the critical role of faecal egg count reduction tests in assessing drug efficacy and detecting resistance.
The strategic use of anthelmintics, such as mebendazole and albendazole, in combination with improved sanitation, health education, and nutritional support, remains central to controlling STH infections. However, the sustainability of these efforts is contingent upon our ability to monitor and manage the emergence of drug resistance, underscored by the importance of faecal egg count reduction tests in ongoing surveillance efforts. This necessitates ongoing research into genetic mechanisms of resistance, the development of novel anthelmintics, and the adoption of integrated control strategies that consider the broader ecological and socio-economic contexts of STH infections.
Ultimately, the fight against STH infections and anthelmintic resistance is a global endeavor that requires the collaboration of researchers, healthcare providers, policymakers, and communities. By fostering innovation in treatment and diagnostic methods, promoting responsible drug use, and supporting public health infrastructure, we can continue to make strides toward the elimination of these infections as public health threats. The journey is complex and fraught with challenges, but with concerted effort and commitment, it is possible to sustain the gains made and move closer to a future free of STH-related morbidity and mortality.